Scientific calendar September 2022
Follicular lymphoma (FL) transformation to B-cell acute lymphocytic leukaemia (B-ALL)
How can complex leukaemia transformations be easily identified?
By evaluating full blood count and morphology information
Using patient history and symptoms only
Using a combination of morphology and immunophenotyping
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Scientific background
Follicular lymphoma (FL) is the most common form of indolent lymphoma in the western world, accounting for approximately 25% of malignant lymphomas [1]. Disease progression of FL is often unpredictable, with 25% – 30% of FL cases progressing – most commonly to diffuse large B cell lymphoma, Hodgkin lymphoma, Burkitt lymphoma and, rarely, to B-lymphoblastic leukaemia/lymphoma (B-ALL) [2]. This phenomenon carries a very poor prognosis, with a life expectancy of approximately ten months post diagnosis [3]. Therefore, detection of disease progression is essential.
Flow cytometry can be used to detect progression of FL to B-ALL.This relies on the ability of the flow cytometer to enable differentiation of the two malignant cell populations.
Case background
A patient with known FL re-presented with a lymph node mass in the right groin and right axilla. A full blood count was performed and the results showed a low haemoglobin with lymphocyte and monocyte populations merging.
A blood smear was performed and blasts plus lymphocytes with cleaved nuclei were observed. The morphological findings concluded that immunophenotyping on a bone marrow aspirate was required.
Immunophenotyping was performed on the incumbent flow cytometer within the laboratory. The results showed expression of blasts and mature B lymphocytes with an overall diagnosis of FL transformation to B-ALL. The sample was then evaluated on a Sysmex flow cytometer; results were shown to be comparable to the predicate.
This case highlights the benefits of flow cytometry in diagnosing intricate cases. It also highlights the importance of including complex disease states when assessing new instrumentation to ensure accurate detection of multiple populations.
These results have been described in a scientific poster [4].
Morphology interpretation
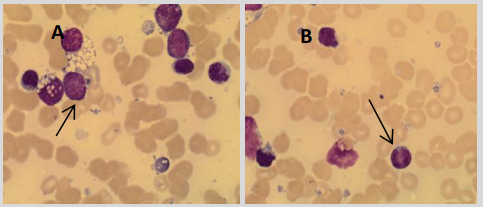
The patient’s bone marrow aspirate was examined and found to have a population of abnormal lymphocytes and blast cells as shown in Figure 1.
Immunophenotyping data interpretation
Immunophenotyping was performed using the following antibodies: CD3, CD7, CD10, CD13, CD14, CD15, CD16, CD19, CD20, CD22, CD33, CD34, CD38, CD45, CD117 and HLA-DR. Cytoplasmic antigen expression was also determined for the following antigens: CD3, CD22, CD79a, MPO and TDT. The data acquisition was performed on a BD FACSCanto II and the Sysmex XF-1600 with 20,000 cells acquired for each tube. Data analysis was performed on BD FACSDiva software and Sysmex XF-1600 software.
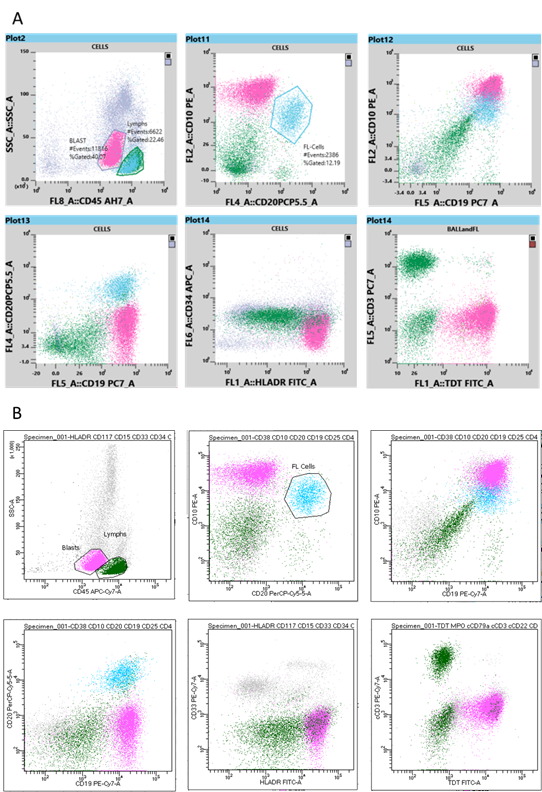
The populations of interest (lymphocytes and blast cells) were gated around based on their SSC and CD45 characteristics allowing for their antigen expression pattern and SI to be determined. Additionally, the CD20 and CD10 dual positive population was gated around in order to identify the FL cells. The gated FL cells were displayed on the following plots: SSCvCD45, CD10vCD20, CD10vCD19 and CD20vCD19. The results from both flow cytometers were then compared.
Immunophenotyping analysis on both flow cytometers allowed identification of a population of blast cells expressing the antigens CD19, HLA-DR, TdT (moderate SI), CD10 (bright SI) and CD45 (dim SI). However, these cells lacked CD20 expression. This immunophenotype is consistent with the World Health Organisation (WHO) criteria for B-ALL [2]. Additionally, both flow cytometers allowed simultaneous identification of a population of mature B lymphocytes which expressed CD20, CD19, CD10 (moderate SI) and CD45 (bright SI), but lacked expression of TdT – an immunophenotype consistent with the previously diagnosed FL. The dot plots from the Sysmex XF-1600 and the BD FACSCanto II are displayed in Figures 2A and 2B, respectively.
Both flow cytometers allowed detection of the two malignant cell populations: the mature B cell population of the previously diagnosed FL and the newly presenting blast cell population of the B-ALL. The immunophenotyping results produced by both the BD FACSCanto II and the Sysmex XF-1600 were comparable with regards to the antigen expression pattern and staining intensity.
References
[1] Ciobanu A, Stanca O, Triantafyllidis I, and Lupu A. (2013): Indolent Lymphoma: Diagnosis and Prognosis in Medical Practice. Maedica; 8(4): 338–342.
[2] Swerdlow S et al. (2017): WHO Classification Of Tumours Of Haematopoetic And Lymphoid Tissues. 4th ed. Lyon: IARC, pp. 266–268.
[3] Morley NJ, Evans LS, Goepel J, and Hancock BW. (2008): Transformed follicular lymphoma: The 25-year experience of a UK provincial lymphoma treatment centre.
[4] Woodhead L and Stevens M. Haematology Department, Royal Hallamshire Hospital, Sheffield Teaching Hospitals NHS Foundation Trust. (2021): Comparing antibody expression and staining intensity between BD FACSCanto II and Sysmex XF-1600 in follicular lymphoma progressing to B-lymphoblastic leukaemia/lymphoma.