HISCL HIT IgG
HIT testing now available your CN-3500/CN-6500
- Integrated chemiluminescent enzyme immunoassay (CLEIA) technology on CN-3500/CN-6500: one platform to test HIT IgG routinely with conventional haemostasis testing
- Fastest automated HIT CLEIA on the market, 24/7, 365 days a year. Reagents, calibrators and controls are all liquid, ready-to-use (LRT) with a long onboard stability of 90 days
- Works with both plasma and serum specimen types. The assay can be integrated into the BloodScience Workcell – a system that can handle EDTA, citrated plasma, and serum samples
- Only a small plasma or serum aspiration volume of 5 µL is required for analysis
- Matched sensitivity and improved specificity to HIT IgG with optimised cut-off 0.6 U/mL with a wide measurement range up to 128.0 U/mL
HIT IgG testing with HISCL CLEIA technology
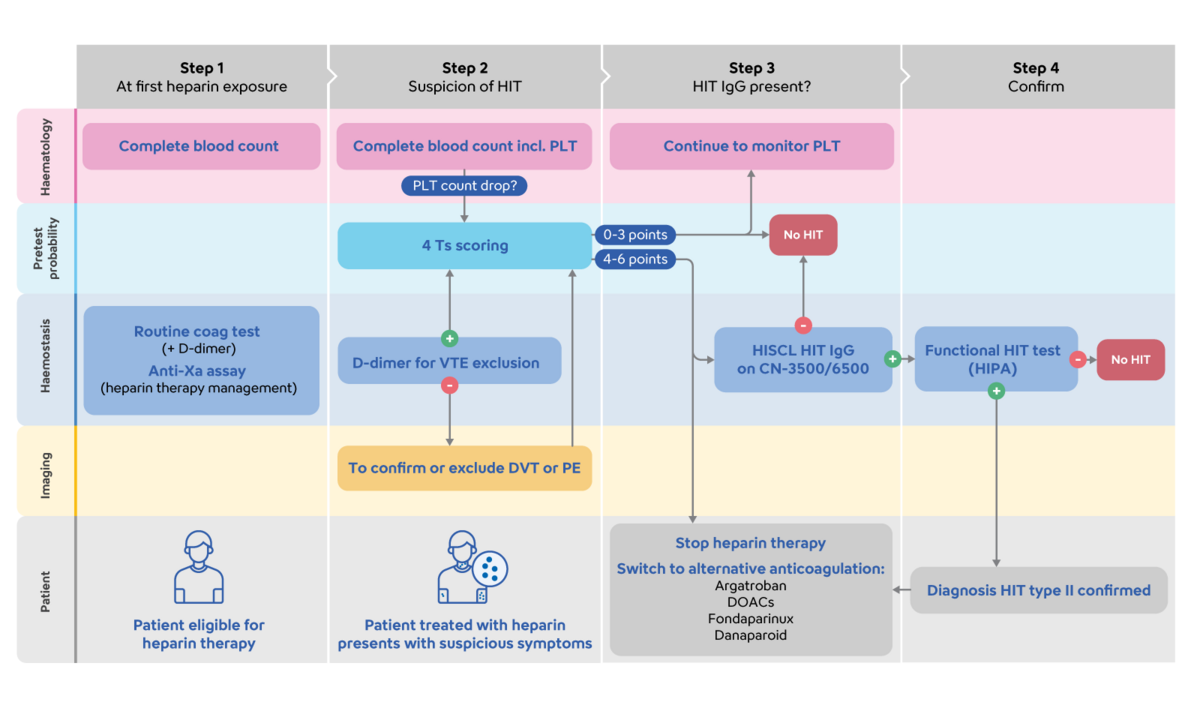
The detection of IgG antibodies against platelet factor 4–heparin complexes is crucial in the diagnosis of HIT type II in combination with a clinical pre-probability 4Ts score. The new HISCL HIT IgG Assay kit* provides a highly sensitive and specific CLEIA for the detection of HIT IgG autoantibodies, with a rapid time to first result.
* The availability of reagents depends on the region
Heparin-induced thrombocytopenia
Heparin-induced thrombocytopenia (HIT) is a severe immune-mediated adverse reaction to heparin anticoagulation therapy, in which autoantibodies (IgG) against complexes of platelet factor 4 (PF4) and heparin are formed. The complex of PF4, heparin and the IgG antibodies activates platelets and triggers thrombin generation, resulting in a highly prothrombotic state. As a consequence, thrombocytopenia and thrombosis are frequently observed, which are associated with significant morbidity and mortality.
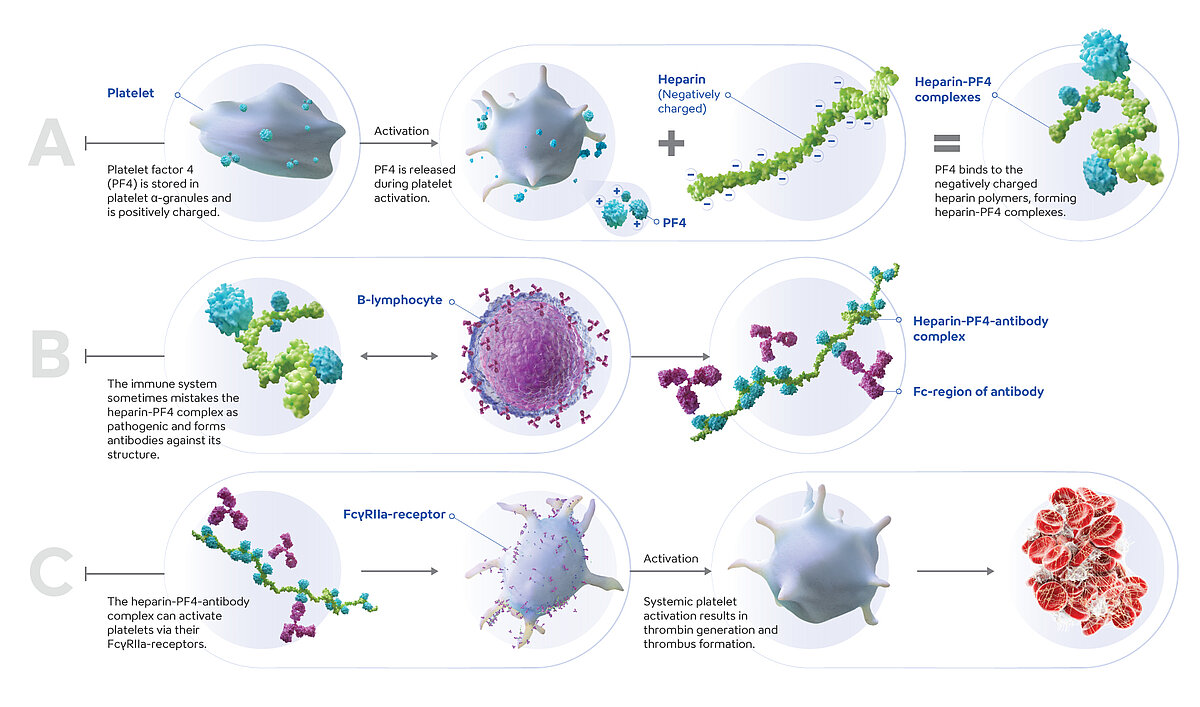
HIT is classified into two types. Type I is a non-immunological response to heparin treatment and a mild platelet aggregation effect, in which the platelet count will normalise even if heparin anticoagulation is continued. Type I typically occurs 48–72 hours post-heparin exposure. Type II HIT is an immune-mediated reaction triggered by heparin anticoagulation therapy and typically occurs within 5–14 days of heparin exposure, with a more pronounced thrombocytopenia and a significantly increased risk of thrombosis.
References
Arachchillage D, Thachil J, Anderson J, Baker P, Poles A, Kitchen S, and Laffan M. Diagnosis and management of heparin-induced thrombocytopenia: Third edition. BJHaem. 2023 Dec; 2024(2): 459-475
HISCL HIT IgG assay principle – powered by CLEIA
The kit detects HIT IgG antibodies in the sample based on a 2-step sandwich CLEIA principle with sample dilution.
- The human PF4 and heparin complex, conjugated with magnetic particles (MP) from the R2 reagent, specifically react with the HIT IgG antibody present in the sample.
- After the B/F (bound and free) separation, alkaline phosphatase (ALP)-labelled and ALP-conjugated anti-human IgG monoclonal antibodies (mouse origin) in the R3 reagent specifically bind to the HIT IgG antibody on the MP.
- After the B/F separation, the ALP-labelled and conjugated antibody on the MP reacts with the CDP-StarR substrate in the R5 reagent to produce a luminescent signal.
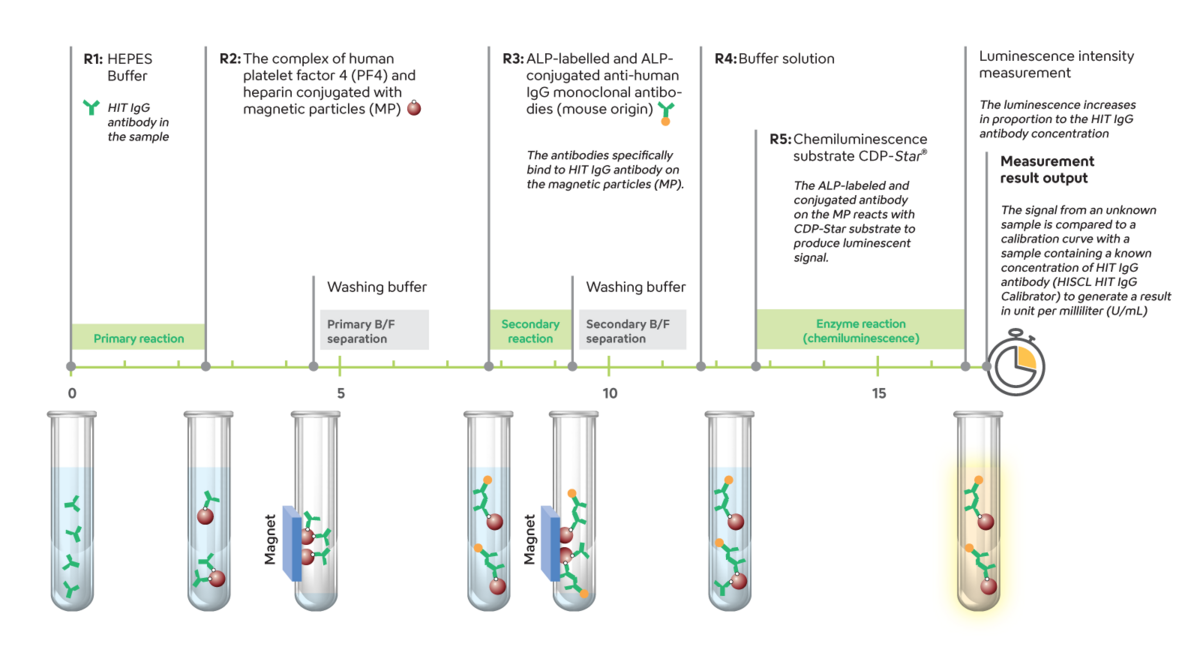
The luminescence increases in proportion to the HIT IgG antibody concentration. The signal from an unknown sample is compared to a calibration curve with a sample containing a known concentration of HIT IgG antibody (HISCL HIT IgG Calibrator) to generate results in units per millilitre (U/mL).
Workflow
Sysmex's products and services support the whole patient journey for HIT diagnosis and management: from the initial indication of falling platelet counts on the Sysmex XN/XR-series, to automated morphology investigation on the Sysmex DI-60, to concurrent coagulation testing on the Sysmex CS/CN-series. We are now proud to introduce the highly sensitive and specific assay to confirm HIT diagnosis, integrated into a single routine coagulometer.
HISCL™ HIT IgG Assay Kit
Measurement item | HIT IgG |
Packaging | 50 tests/package |
Measurement principle | Chemiluminescent enzyme immunoassay (CLEIA) |
Purpose of use | Measurement of IgG antibodies to platelet factor 4 (PF4)- heparin complexes (HIT IgG antibodies) |
Sample type | Human citrate plasma and serum |
Measurement range | Plasma: 0.10-128.00 U/mL Serum: 0.10-108.3 U/mL |
Cut off value | 0.6 U/mL |
Analysis time | 17 minutes (from the first measurement) |
Applicable instruments | Sysmex Automated Blood Coagulation Analyser CN-3500/6500 |
On-board stability | 90 days |
Sysmex UK LTD
Sysmex House
Garamonde Drive
Wymbush
Milton Keynes
MK8 8DF
0333 320 3460
CN-3500/CN-6500 complete online training - Begin your journey to expertise with Sysmex CN-3500 and CN-6500 analysers
For user trainings, please contact your local Sysmex representative.
Product documents
Regulatory Documents
Regulatory documents, such as Instructions for Use, can be accessed with a valid My Sysmex login:
Go to My Sysmex
